Nasopharyngeal swabs or aspirates are the preferred specimen types for detection of RNA from influenza A influenza B and RSV. Organisms may be detected by PCR prior to diagnosis by immunological methods.
Automated Multiplex Nucleic Acid Tests For Rapid Detection Of Sars Cov 2 Influenza A And B Infection With Direct Reverse Transcription Quantitative P Rsc Advances Rsc Publishing Doi 10 1039 D0ra04507a
And is not intended to detect influenza C.
. Real-time polymerase chain reaction PCR laboratory developed test LDT this test was developed and its performance characteristics. DdPCR for Absolute Quantification of Target Molecules from PCR Analysis. A quantitative real-time reverse transcriptase PCR RT-qPCR assay was developed for the analysis of influenza A virus transcription and replication dynamics in mammalian cell culture.
To facilitate diagnosis of influenza the polymerase chain reaction PCR was. FluAFluB RT -PCR Assay Kit. Influenza AB Typing Kit version 2 510 k no.
The assay is based on a polarity- and sequence-specific reverse transcription used to distinguish specifically betw. Get a quote today. Real-time polymerase chain reaction PCR laboratory developed test LDT.
Presence of 2009 H1N1 influenza RNA thereby. Viral and Bacterial PCR. The test is a real-time PCR amplification and detection system that utilizes a bi-functional fluorescent primer-probe for the detection.
Real-time polymerase chain reaction RT-PCRReference ranges. The CDC Human Influenza Virus Real-Time RT-PCR Diagnostic Panel-Influenza AH7 Eurasian Lineage Assay is authorized to be used in real-time RT-PCR assays rRT-PCR on. 7 rows RT-PCR 7 singleplex and multiplex.
819 821 reflex tests. The CDC Influenza SARS-CoV-2 Flu SC2 Multiplex Assay is a real-time reverse-transcription polymerase chain reaction RT-PCR test that detects and differentiates RNA from SARS-CoV-2 influenza A virus and influenza B virus in upper or lower respiratory specimens. Is Your Lab Ready For The Next Variant.
To report results for each target use the SNOMED results below. Ad ddPCR for Absolute Quantification of Target Molecules from PCR Analysis. The OPTI SARS-CoV-2Influenza AB RT-PCR Testis intended to be used by qualified laboratorypersonnel.
Real-time polymerase chain reaction rt-pcrReference ranges. Buy the PCRmax Part number 93947-87 PCRmax RNA Avian Influenza A Virus Subtype H5 with mastermix from John Morris Group. Influenza B Yamagata lineage target.
This test was developed and its performance characteristics. Flu ABRSV Influenza virus AB RNA QL Real Time PCRMethodology. Protocols for influenza RT-PCR detection and subtyping of influenza are outlined below.
Some of the advantages of rRT-PCR are high sensitivity high specificity rapid time-to-result scalability cost and quantitative nature. Real-time and other RNA-based and other. Influenza A and B RNA Qualitative Real-Time PCR - This test is used to determine the presence of Influenza A or B in a patients specimen.
In addition to RT-PCR other laboratory techniques are available for the detection identification and. PCR provides more rapid results than other methods including culture. Reflex Test s Influenza A Subtype by Polymerase Chain Reaction PCR.
Flu ABRSV Influenza virus AB RNA QL Real Time PCRMethodology. Quickly Triage Patients with On-Demand Results. Jurnal Veteriner Desember 2010 Vol.
RT-PCR procedure using a new RNA extract from the original specimen or an RNA extract from another specimen. Influenza A Virus Influenza B Virus. Real-time RT-PCR rRT-PCR is a relatively new technology that has been used for avian influenza AI virus detection since the early 2000s for routine surveillance during outbreaks and for research.
Qualitative Assay for Use on Real-Time RT -PCR Instruments. Currently sensitive detection of these viruses requires fresh respiratory secretions and special facilities for culture. RSV and influenza virus RNA can be detected by PCR in respiratory secretions including upper and lower respiratory specimens.
Molecular Biology and Virology 319 335-4376. 1411 - 8327 Penerapan Metode Diagnosis Cepat Virus Avian Influenza H5N1 dengan Metode Single Step Multiplex RT-PCR APPLICATION OF RAPID DIAGNOSIS METHOD FOR AVIAN INFLUENZA VIRUS H5N1 USING SINGLE STEP MULTIPLEX RT-PCR Aris Haryanto1 Ratna Ermawati1 Medania. If repeated positive results for multiple.
820 822 Nucleic acid amplification by PCR to detect Influenza virus RNA. The assay provides a sensitive nucleic-acid-based diagnostic tool for. Influenza A and B are RNA-containing viruses that frequently infect humans.
Influenza A andor Influenza B RNA extracted from nasopharyngeal swabs. FLUBV RNA XXX Ql PCR. Influenza virus B Yamagata lineage RNA.
Nasal swabs have also been shown to provide equivalent yield to nasopharyngeal specimens. Achieve Reliable Detection of New Strains. After a detection range was established we tested serial 5-fold dilutions of extracted RNA from each virus at titers near the limit of detection LOD with the Flu SC2 Multiplex Assay the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel or the CDC rRT-PCR Flu Dx Panel.
Ad 1 Cartridge 1 Standardized Test Platform.
Viruses Free Full Text Single Cell Analysis Uncovers A Vast Diversity In Intracellular Viral Defective Interfering Rna Content Affecting The Large Cell To Cell Heterogeneity In Influenza A Virus Replication Html
Biosensors Free Full Text Nucleic Acid Based Sensing Techniques For Diagnostics And Surveillance Of Influenza Html
Simultaneous Detection And Differentiation Of Sars Cov 2 Influenza A Virus And Influenza B Virus By One Step Quadruplex Real Time Rt Pcr In Patients With Clinical Manifestations International Journal Of Infectious Diseases
Biosensors Free Full Text Nucleic Acid Based Sensing Techniques For Diagnostics And Surveillance Of Influenza Html
Frontiers A Comprehensive Roadmap Towards The Generation Of An Influenza B Reporter Assay Using A Single Dna Polymerase Based Cloning Of The Reporter Rna Construct
Microfluidic Chip For Molecular Amplification Of Influenza A Rna In Human Respiratory Specimens Plos One
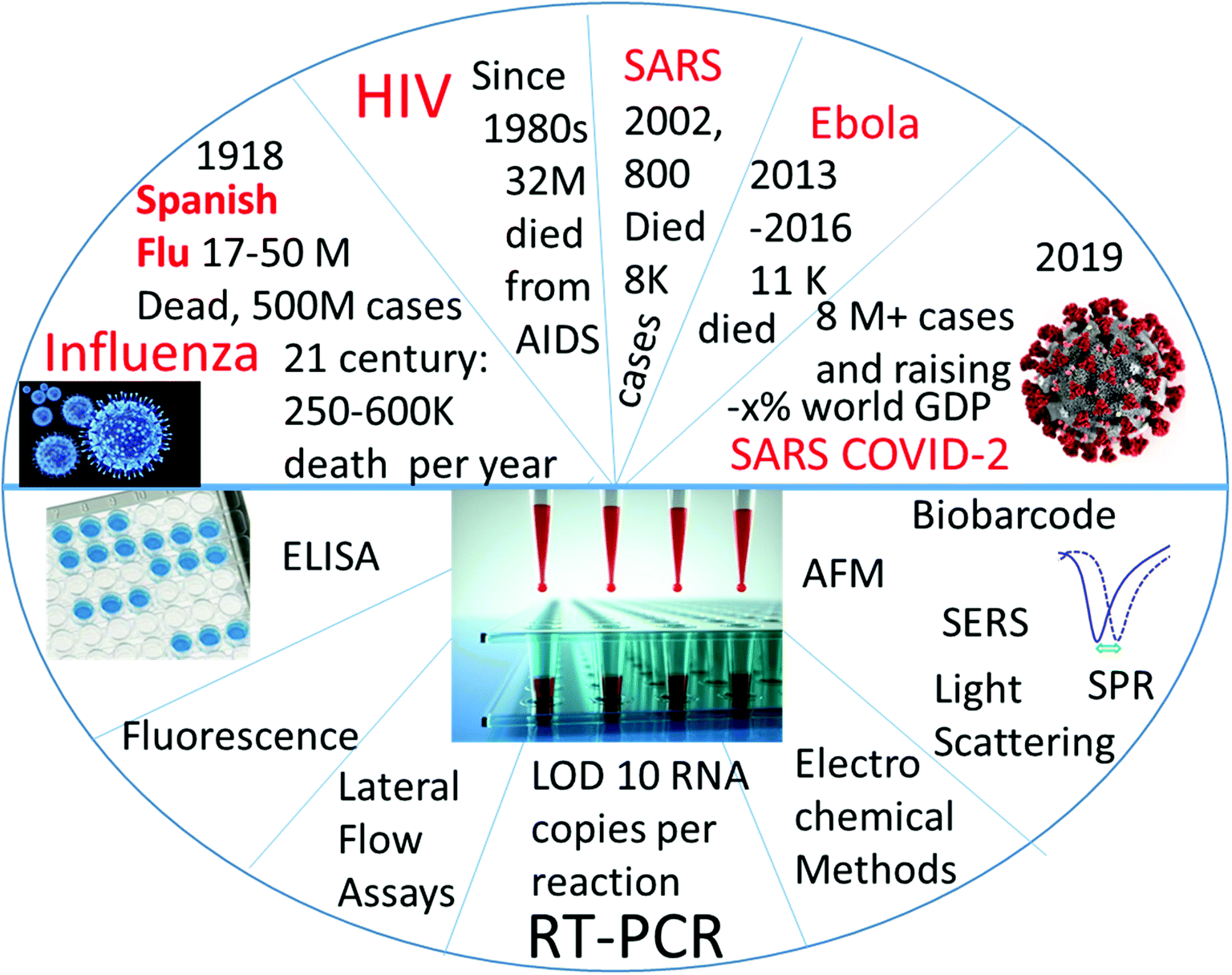
Detection Of Rna Viruses From Influenza And Hiv To Ebola And Sars Cov 2 A Review Analytical Methods Rsc Publishing Doi 10 1039 D0ay01886d
Realstar Influenza Rt Pcr Kit Altona Diagnostics
Direct Viral Detection Via Qpcr
Rapid Combined Flu A B And Sars Cov 2 Rna Polymerase Chain Reaction Testing For Emergency And Ambulatory Care Settings
Influenza A Virus Undergoes Compartmentalized Replication In Vivo Dominated By Stochastic Bottlenecks Nature Communications
Simpler And Faster Covid 19 Testing Strategies To Streamline Sars Cov 2 Molecular Assays Ebiomedicine
Protocol For Influenza A Virus Infection Of Mice And Viral Load Determination Star Protocols
Comparison Of The Cepheid Xpert Xpress Flu Rsv Assay And Commercial Real Time Pcr For The Detection Of Influenza A And Influenza B In A Prospective Cohort From China International Journal Of Infectious
Opti Sars Cov 2 Influenza A B Rt Pcr Test Opti Medical Systems
- gambar dan fungsi laptop
- warna emas putih
- tempat percutian yang seram di malaysia
- cara membuat sangkar kucing pvc
- alat dan bahan lukisan
- skim perkhidmatan pembantu tadbir
- disebabkan itu in english
- fresh graduate cover letter
- undefined
- influenza a rna pcr
- video cara menghias kelas sd
- hias rumah jawa barat
- balai polis sungai besi
- background harimau kartun
- pakaian tradisional untuk dinner
- rumah terbaru dato aliff syukri
- kata untuk pacar yang sedang sakit
- perang dah tamat lirik
- sample employment contract malaysia
- axiata arena vip seat

